.
Quality Policy:
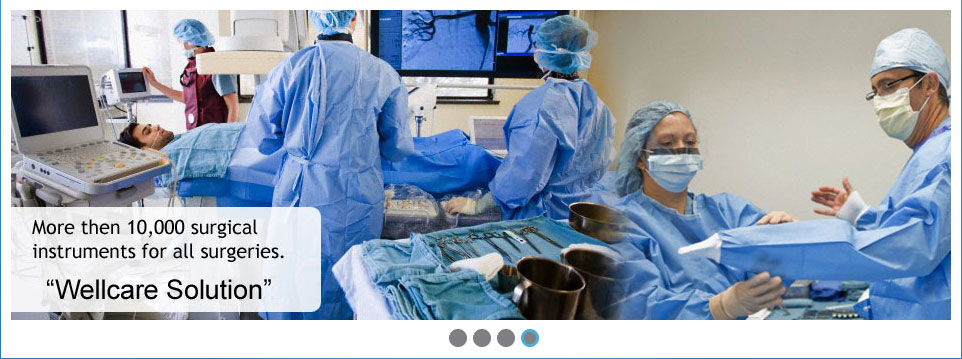
With over many years' experience, "Wellcare" is an industry leader in offering OEM solutions to some of the largest medical device companies in the world. From concept design through to post-market, "Wellcare" delivers a full service OEM product development and manufacturing partnership. Whatever your needs, challenges or ideas, Wellcare's expert team can find a solution customized to your needs.
Concept Design Prototyping:
Our concept design team works with you to develop blue sky ideas according to individual customer requirements. The designers are put through the same core skills surgical training exercises as surgeons, allowing them to truly immerse themselves in the role of the end user.
Product Development Engineering:
Our product development team takes a working prototype from concept and manages your project through to production. This involves expert planning, refining designs to the highest standards ensuring they are effectively engineered for manufacture, and gaining clinical feedback every step of the way.
Licensing and Regulatory Services:
Our medical devices comply with international standards and our regulatory teams are always on hand to guide you all the way from submission to clearance, while also offering product support services. This includes:
- EU technical file for CE marking approval
- All other international device approvals and registrations
Added Value Manufacture:
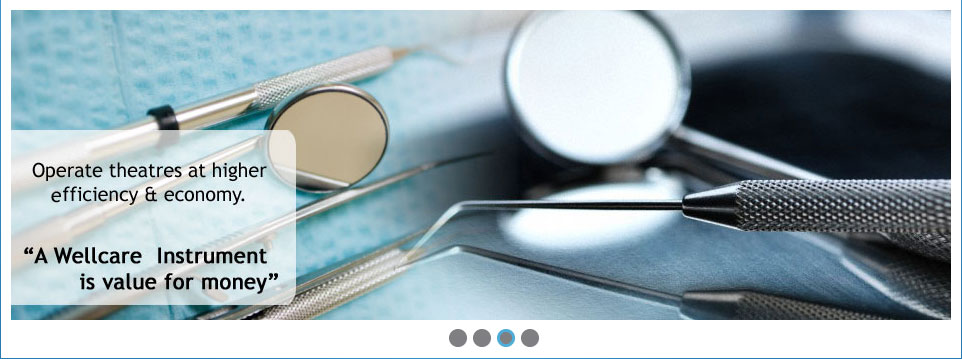
Wellcare's in-house manufacture facility is made up of machine shop, assembly, clean room and injection moldings. Our state-of-the-art machinery allows us to produce expert precision-engineered products in close collaboration with our product design and quality teams. From small batch runs through to volume production, our manufacture and assembly teams ensure our devices meet exacting quality specifications.
Post-Market Communications:
Our communications experts are dedicated to helping you with all of your post-market needs. This includes your IFU (instructions for use), labeling and packaging requirements in addition to branding of the device itself.